Content CAS ETH in Regulatory Thinking
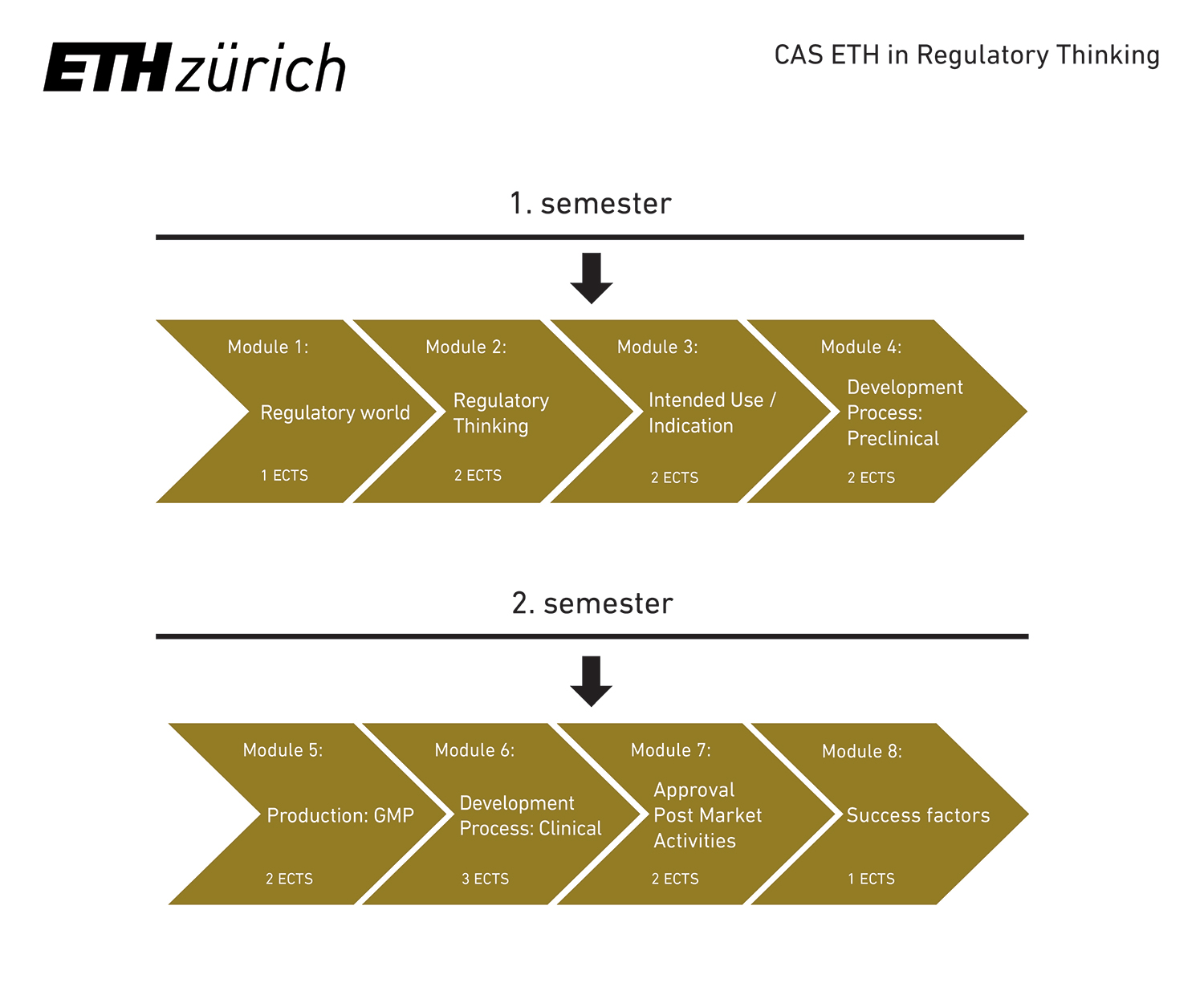
Modules
Regulatory Thinking (RT) is a methodological thinking approach. RT will be applied to the research and development efforts to ensure that the most promising research results can be translated into clinical applications. Regulatory Thinking is part of a company’s business strategy that considers the interests of the various stakeholders in medical technology, in vitro diagnostics, pharmaceuticals and biotechnological products, including future technologies.
The CAS ETH RT consists of the following modules:
Regulatory World
- Stakeholders: From patient to payors
- Regulatory Landscape
- Development process
Regulatory Thinking
- Interaction with the Regulator
- Regulations / Directives / Laws / Guidelines
- Safety / Efficacy / Performance / Transparency
- QMS
Intended Use / Indication
- Medical need assessment
- Intended Use / Indication
- Development plan
- Risk assessment
Development Process: Preclinical
- GLP
- Preclinical Safety & Efficacy Assessment
- Starting dose (first in human)
Production / GMP
- GMP
- Scaling up process
- Drug Quality
Development Process: Clinical
- GCP / Ethics
- Phase I, II, III
- Submission to ethic committee, regulator
Approval / Post Market Activities
- Market Access
- HTA / Vigilance / Pricing / Reimbursement
- Lifecycle Management
Success Factors
- Discuss own projects
Contact CAS ETH in Regulatory Thinking:
Medizinausbildung ETH
Leopold-Ruzicka-Weg 4
8093
Zürich
Switzerland

Get in touch and book your personal consultation on the MAS ETH in digital Clinical Research:
Professur Translationale Ern.biol.
Schorenstrasse 16
8603
Schwerzenbach
Switzerland
